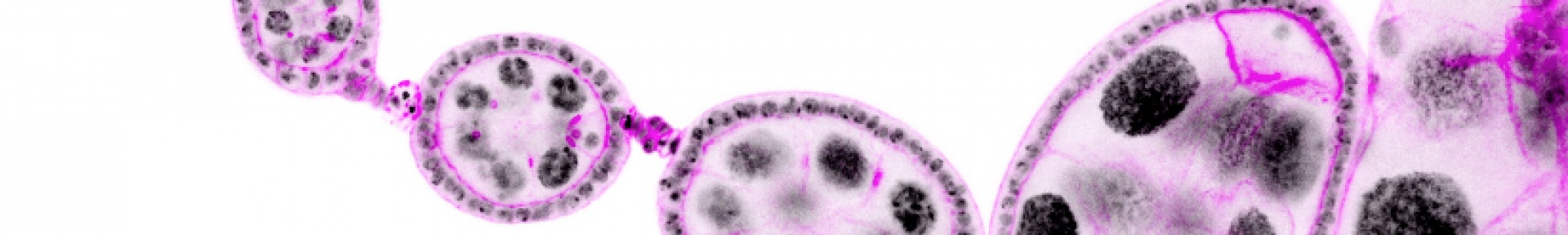
The living world has an infinite variety of shapes and sizes at the scale of whole organisms and this diversity contributes to our wonder for nature. Nevertheless, the existence of this diversity is not based on aesthetic criteria but on functional constraints. This is the case with the neck of the giraffe, reaching the highest leaves, or the fins of the flying fish mimicking the wing of the birds. The same diversity of forms, based on the same functional constraints, exists also at the level of our organs. For example, the tree structure of our lungs maximizes the exchange surface between air and blood to facilitate gas exchange. It is therefore important to understand how our genes sculpt these organs into a specific form (morphogenesis) and with an appropriate size (growth). The study of mechanisms controlling growth and morphogenesis is based on dynamic multi-scale biological processes (molecular, cellular and tissular) and requires approaches involving developmental biology, genetics, cell biology and biophysics.
In addition to these basic aspects, our work is also medically relevant since 80% of cancers have an epithelial origin and deregulation of the mechanisms controlling their growth or their morphogenesis are involved in tumor development. In fact, many of the genes we are studying are tumor suppressors or oncogenes in humans. In addition, some of these genes are also involved in dystrophies in humans - including the gene encoding dystrophin, involved in Duchenne and Becker dystrophies - and their study in our system sheds a new light on their involvement in these genetic diseases.
Funders

Research
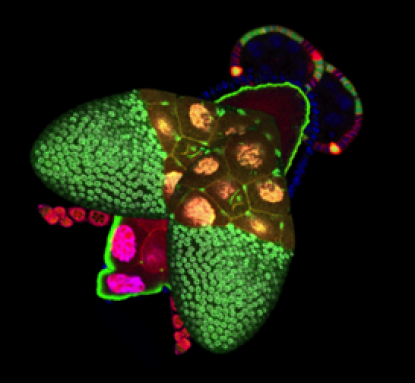
These mechanisms controlling growth and morphogenesis are easier to study in epithelium because epithelial cells have a relatively simple and very stereotyped shape. Thus, any defect in shape or size is easily observable and computable. In addition, most of our organs are made up of this cell type, characterized by closely juxtaposed cells, polarized, associated by intercellular junctions and resting on a basal lamina. We chose Drosophila (fruit fly) as a model organism, for the unequaled possibilities it offers in terms of genetic tools and cellular imaging. Specifically, we use follicular cells of the Drosophila ovaries as an in vivo epithelium model. The ovarian follicular epithelium has a morphology very similar to mammalian epithelia and a rapid but strictly controlled growth. Anatomically, this epithelium surrounds the germ cells within the reproductive organ, forming ‘ovarian follicles’ or ‘egg chambers’, precursors of the future egg. Interestingly, the elongation of the ovarian follicle, which progressively matures from a spherical to an elongated form, is generated by the follicular epithelium.
Thus, the projects developed within the team focus on two axes:
1. Understand the growth coordination mechanisms between two adjacent tissues. For this, we analyze the modes of communication between the germ cells and the follicular cells, allowing the synchronization of their growth during the development of the ovarian follicles and thus ensuring the homeostasis of the reproductive organ.
2. Understand the morphogenetic mechanisms leading to elongation of ovarian follicles. We study on the one hand F-actin cytoskeleton function and dynamics at the apical domain and cell junctions of follicular epithelial cells, in contact with the germ cells during the early elongation of the follicles. On the other hand, we analyze the involvement of the Dystrophin-Dystroglycan protein complex and the basal domain of the epithelium in the later follicle elongation phase and especially its implication in planar cell polarity and extracellular matrix organization.
Members
Publications
The transcription factor Traffic jam orchestrates the somatic piRNA pathway in Drosophila ovaries.
Published on 22 Apr 2025 in Cell reports , vol. 44 - pp 115453
Alizada A, Martins A , Mouniée N, Rodriguez Suarez JV, Bertin B , Gueguen N , Mirouse V , Papameletiou AM, Rivera AJ, Lau NC, Akkouche A , Maupetit-Méhouas S, Hannon GJ, Czech Nicholson B, Brasset E
Gap junctions allow transfer of metabolites between germ cells and somatic cells to promote germ cell growth in the Drosophila ovary.
Published on 18 Feb 2025 in PLoS biology , vol. 23 - pp e3003045
Vachias C , Tourlonias C , Grelée L , Gueguen N , Renaud Y , Venugopal P , Richard G , Pouchin P , Brasset E , Mirouse V
The Dystrophin-Dystroglycan complex ensures cytokinesis efficiency in Drosophila epithelia.
Published on 15 Nov 2024 in EMBO reports
Gonçalves M, Lopes C, Alégot H , Osswald M, Bosveld F, Ramos C, Richard G , Bellaiche Y, Mirouse V , Morais-de-Sá E
The transcription factor Traffic jam orchestrates the somatic piRNA pathway in Drosophila ovaries
Published on 12 Sep 2024 in bioRxiv
Alizada A*, Martins A* , Mouniée N* , Rodriguez Suarez JV*, Bertin B , Gueguen N , Mirouse V , Maupetit-Mehouas S , Rivera AJ, Lau NC, Hannon GJ, Czech Nicholson B, Brasset E.
Basement membrane diversification relies on two competitive secretory routes defined by Rab10 and Rab8 and modulated by dystrophin and the exocyst complex.
Published on 04 Mar 2024 in PLoS genetics , vol. 20 - pp e1011169
Evolution and developmental functions of the dystrophin-associated protein complex: beyond the idea of a muscle-specific cell adhesion complex.
Published on 13 Jun 2023 in Frontiers in cell and developmental biology , vol. 11 - pp 1182524
In preprints: get to know your neighbours – cell interface surveillance through a molecular zip code.
Published on 15 Apr 2023 in Development (Cambridge, England) , vol. 150
Ríos-Barrera LD, Mirouse V
Multiple functions of the scaffold protein Discs large 5 in the control of growth, cell polarity and cell adhesion in Drosophila melanogaster.
Published on 18 Jun 2020 in BMC developmental biology , vol. 20 - pp 10
Venugopal P , Veyssière H , Couderc JL , Richard G , Vachias C , Mirouse V
Sequential Ras/MAPK and PI3K/AKT/mTOR pathways recruitment drives basal extrusion in the prostate-like gland of Drosophila.
Published on 08 May 2020 in Nature communications , vol. 11 - pp 2300
Rambur A , Lours-Calet C, Beaudoin C , Buñay J, Vialat M , Mirouse V , Trousson A , Renaud Y , Lobaccaro JA, Baron S , Morel L , de Joussineau C
Oriented basement membrane fibrils provide a memory for F-actin planar polarization via the Dystrophin-Dystroglycan complex during tissue elongation.
Published on 08 Apr 2020 in Development (Cambridge, England) , vol. 147
Cerqueira Campos F , Dennis C , Alégot H , Fritsch C , Isabella A, Pouchin P , Bardot O , Horne-Badovinac S, Mirouse V
Jak-Stat pathway induces Drosophila follicle elongation by a gradient of apical contractility.
Published on 08 Feb 2018 in eLife , vol. 7
Drosophila LKB1 is required for the assembly of the polarized actin structure that allows spermatid individualization.
Published on 02 Aug 2017 in PloS one , vol. 12 - pp e0182279
Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine.
Published on 13 Aug 2014 in BMC genomics , vol. 15 - pp 679
Borrel G, Parisot N, Harris HM, Peyretaillade E, Gaci N, Tottey W, Bardot O , Raymann K, Gribaldo S, Peyret P, O'Toole PW, Brugère JF
ScientiFig: a tool to build publication-ready scientific figures.
Published on 30 Nov 2013 in Nature methods , vol. 10 - pp 1048
Genome Sequence of “Candidatus Methanomassiliicoccus intestinalis” Issoire-Mx1, a Third Thermoplasmatales-Related Methanogenic Archaeon from Human Feces.
Published on 11 Jul 2013 in Genome announcements , vol. 1
Borrel G, Harris HM, Parisot N, Gaci N, Tottey W, Mihajlovski A, Deane J, Gribaldo S, Bardot O , Peyretaillade E, Peyret P, O'Toole PW, Brugère JF
PTEN controls junction lengthening and stability during cell rearrangement in epithelial tissue.
Published on 10 Jun 2013 in Developmental cell , vol. 25 - pp 534-46
Bardet PL, Guirao B, Paoletti C, Serman F, Léopold V, Bosveld F, Goya Y, Mirouse V , Graner F, Bellaïche Y
Tissue-specific function of Patj in regulating the Crumbs complex and epithelial polarity.
Published on 30 Dec 2012 in Development (Cambridge, England) , vol. 139 - pp 4549-54
Pénalva C, Mirouse V
DroPNet: a web portal for integrated analysis of Drosophila protein-protein interaction networks.
Published on 30 Jul 2012 in Nucleic acids research , vol. 40 - pp W134-9
Renaud Y , Baillif A, Perez JB, Agier M, Mephu Nguifo E, Mirouse V
The LKB1/AMPK polarity pathway.
Published on 06 Apr 2011 in FEBS letters , vol. 585 - pp 981-5
Mirouse V , Billaud M
A two-step Notch-dependant mechanism controls the selection of the polar cell pair in Drosophila oogenesis.
Published on 30 Aug 2010 in Development (Cambridge, England) , vol. 137 - pp 2703-11
Vachias C , Couderc JL , Grammont M
Bazooka is required for polarisation of the Drosophila anterior-posterior axis.
Published on 30 May 2010 in Development (Cambridge, England) , vol. 137 - pp 1765-73
Doerflinger H, Vogt N, Torres IL, Mirouse V , Koch I, Nüsslein-Volhard C, St Johnston D
aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells.
Published on 30 Apr 2010 in Cell , vol. 141 - pp 509-23
Morais-de-Sá E, Mirouse V , St Johnston D